|  Washington,
D.C., June 23, 2003---The
Prostate Cancer Prevention Trial (PCPT) was stopped early ‘in
the public interest’, according to trial sponsors at the National
Cancer Institute in an early morning press conference. The Merck
drug finasteride (PROSCAR) was found to reduce prostate cancer occurrence
by 25% compared to placebo in men who participated in the 7-year NCI
study. Coincident with the NCI announcement, the New England Journal
of Medicine deemed the results of “critical importance”
and published findings
of the study in an early release on the NEJM website. The article
is of keen interest, because with Washington,
D.C., June 23, 2003---The
Prostate Cancer Prevention Trial (PCPT) was stopped early ‘in
the public interest’, according to trial sponsors at the National
Cancer Institute in an early morning press conference. The Merck
drug finasteride (PROSCAR) was found to reduce prostate cancer occurrence
by 25% compared to placebo in men who participated in the 7-year NCI
study. Coincident with the NCI announcement, the New England Journal
of Medicine deemed the results of “critical importance”
and published findings
of the study in an early release on the NEJM website. The article
is of keen interest, because with  these
data, finasteride (PROSCAR) becomes the first thoroughly-tested agent
shown to help prevent a disease which is expected to take the lives
of nearly 30,000 American men this year. these
data, finasteride (PROSCAR) becomes the first thoroughly-tested agent
shown to help prevent a disease which is expected to take the lives
of nearly 30,000 American men this year.
“Millions of men may benefit from finasteride’s ability
to reduce prostate cancer risk,” said Leslie Ford, M.D., who oversaw
PCPT for the Institute. The nation’s number one cancer doctor,
Andrew C. von Eschenbach, Director of the NCI, called it a “landmark
study.” The story appeared on the front
page of the New York Times, and it was widely covered by other major
news agencies such as CNN
and The Wall
Street Journal. At the press conference, which will be available
online as a webcast for the next year, Dr. Peter Greenwald, Director
of Cancer Prevention for the NCI, said, “This trial proves that
prostate cancer is at least in part preventable. It is a huge step forward.”
On the day
of the NCI press conference, USRF Director Leonard S. Marks, M.D., appeared
live on CNN television to explain the importance of PCPT. 
 On
a cautionary note, Ian Thompson, M.D., principle investigator of PCPT,
said, “Men in the study who developed prostate cancer while taking
finasteride were more likely to have high-grade cancers, which, when
found in the general population, may spread quickly even if the tumors
are small. But, more than 97 percent of men who did develop prostate
cancer during this study had early-stage cancers, which are most often
curable.” The significance of this cautionary note cannot be known
until information about the biological behavior of these high-grade
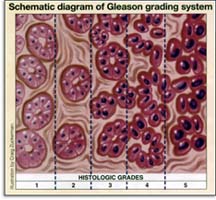
cancers becomes available. The Gleason Grading System for prostate cancer
is explained here. On
a cautionary note, Ian Thompson, M.D., principle investigator of PCPT,
said, “Men in the study who developed prostate cancer while taking
finasteride were more likely to have high-grade cancers, which, when
found in the general population, may spread quickly even if the tumors
are small. But, more than 97 percent of men who did develop prostate
cancer during this study had early-stage cancers, which are most often
curable.” The significance of this cautionary note cannot be known
until information about the biological behavior of these high-grade
cancers becomes available. The Gleason Grading System for prostate cancer
is explained here.
The reason men on finasteride had more high-grade tumors is currently
unknown, but the researchers are studying several possibilities. The
drug affects the appearance of prostate cancer cells, and this may lead
to a false estimate of tumor grade, which is determined visually by
a pathologist. Another possible explanation being examined is whether
finasteride truly causes more aggressive tumors to develop—either
by preventing only low-grade tumors, or by making the prostate gland
more favorable to aggressive tumors. A
USRF study examining the long-term effects of finasteride on prostate
tissue, determined by serial biopsy, was published in 1999.
 PCPT,
which was a $73 million project, is the most important prostate cancer
chemoprevention trial ever completed. The trial was coordinated for
the NCI by the Southwest
Oncology Group, which is one of the world’s largest clinical
trials organization. The first man was enrolled in October of 1993,
and the trial was stopped on March 3, 2003. At 221 study sites across
the nation, approximately 25,000 men were enrolled and 18,900 completed
randomized treatment until the study was stopped 15 months early. By
the close of the study, prostate cancer had been found in PCPT,
which was a $73 million project, is the most important prostate cancer
chemoprevention trial ever completed. The trial was coordinated for
the NCI by the Southwest
Oncology Group, which is one of the world’s largest clinical
trials organization. The first man was enrolled in October of 1993,
and the trial was stopped on March 3, 2003. At 221 study sites across
the nation, approximately 25,000 men were enrolled and 18,900 completed
randomized treatment until the study was stopped 15 months early. By
the close of the study, prostate cancer had been found in  about
18 percent of the men who took finasteride, or 803 men out of 4,368.
About 24 percent of men who took placebo, or 1,147 men out of 4,692,
also had been diagnosed with prostate cancer. Many of the men with cancer
had normal prostate exams and PSA levels, and the disease was found
only because the trial required an end-of-study biopsy. Details of the
study are available in the NEJM publication
and also on this NIH
website. The key figure
of PCPT is shown here. about
18 percent of the men who took finasteride, or 803 men out of 4,368.
About 24 percent of men who took placebo, or 1,147 men out of 4,692,
also had been diagnosed with prostate cancer. Many of the men with cancer
had normal prostate exams and PSA levels, and the disease was found
only because the trial required an end-of-study biopsy. Details of the
study are available in the NEJM publication
and also on this NIH
website. The key figure
of PCPT is shown here.
Three other large, long-term prostate cancer prevention trials are
currently in the enrollment phase:
(1) a study undertaken by GlaxoSmithKline involving the drug dutasteride
(AVODART).
(2) a study undertaken by Merck involving the drug rofecoxib (VIOXX).
(3) the SELECT
trial undertaken by the NCI, involving selenium and vitamin E.
| Enrollment in the AVODART and VIOXX trials
is underway at Urological Sciences Research Foundation (USRF)
in Los Angeles and other sites across the U.S. For information
about these trials, interested parties may call USRF offices (Dr.
Macairan), 310-838-6347. |
|







